It is additionally imperative that you enhance This system for initialization soon after Every single operate and before likely for another injection. This system for initialization shall be optimized this kind of that there shall be no carry-over to the next operate plus the system stabilizes with First composition prior to the following injection.
A cookie is a small information and facts file that is definitely saved on your Laptop, smartphone or pill each and every time you stop by our Web-site. Some cookies are ours and Other people belong to exterior firms that give services for our Internet site.
So you realize it in a very quite simple way, we provides you with a fictitious example: if your previous searches on the net ended up relevant to suspense literature, we would explain to you promotion for suspense textbooks.
Detection and identification of this sort of species are critical simply because they can have an impact on the efficacy and basic safety of formulated biopharmaceuticals.
Although excellent validation practices are explained in ICH Q2, this document isn't going to depth the sensible implications for validation; for example, just a few details are involved regarding experimental design and style and statistical details treatment method.
. Thus, when working for a very long time, the coil heat is much more severe. The technique designed Within this research was utilized for in vitro
The crystals form underneath intense heat, And exactly how speedily they interesting was shown to ascertain their stage. To exhibit, the scientists showed they could switch phases on and off by reheating crystals and making it possible for them to chill for both lengthier or shorter amounts of time. The result is really a alter in the crystalline symmetry that dictates the electronic topology. Determine courtesy of Han Wu/Yi Research Team/Rice University.
Thus, increasing the general width from the multi-coil framework supplies many Gains: (i) it expands the shipping and delivery selection of the magnetic drug and increases its residence time while in the magnetic field; (ii) it swiftly boosts the delivery pace but slows down its acceleration near the focus on spot, making it simpler for it to stay inside the concentrate on place; and (iii) it improves the aggregation location of your magnetic drug, allowing larger sized tumors for being handled with just one software, which is amazingly valuable for magnetic focusing on therapy. In addition, as the overall width on the multi-coil composition increases, the powerful number of the magnetic area also extends. This results in the magnetic drug shifting in direction of the concentrate on area about a higher length, causing reduce drug concentrations in remote usual tissues and higher concentrations in the concentrate on space. Due to this fact, the therapeutic result is Improved, and poisonous Unintended effects over the organism are correctly lessened.
Accelerating procedure development pursuits is critical for remaining competitive within the pharmaceutical marketplace now and calls for potent collaboration with analytical method development endeavours. As a deal development and production Firm (CDMO) dedicated to supplying Sophisticated method development abilities, GSK Biopharmaceuticals has recognized a remarkably seasoned analytical team focused on supporting system development with the specific goal of enabling the quick scale-up and tech transfer of biopharma partner procedures.
In one case, silicone was detected in an item right after its container was improved. The first release sizing exclusion method was insufficient since the silicone peak interfered Together with the detection of protein-connected impurities. To beat the trouble, a method was produced that bound the silicone into the chromatography column while the protein was permitted to pass through and be analyzed.
animal or human experiments would require greater magnetic fields and may hence have additional cooling links or be crafted from superconducting components instead.
The steadiness of analytical options (sample or standard) might be founded on car-injector for at least twelve several hours consistently inside of a sequence method to check here understand The soundness of all elements and ruggedness of the method (peak shapes, column back again force more than the stretch of time).
The magnetic discipline power and gradient could possibly be efficiently enhanced and flexibly altered by changing the volume of excitation coils or rising the amount of turns of winding coil, the volume of electric power materials, and the availability recent. This allows for specific focused therapy for tiny tumors whilst further more cutting down the size of your goal area.
Just after separation of all impurities and degradation merchandise, absorption spectra of many of the compounds are recorded and in more info comparison by using overlay spectra of all acknowledged impurities together with the principal analyte in Every single pressure situation and finalizing a wavelength where by all impurities are detected and quantified and possess the most absorbance. Just in case this isn't possible, choose distinct wavelengths to estimate all impurities.
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Brian Bonsall Then & Now!
Brian Bonsall Then & Now! Lark Voorhies Then & Now!
Lark Voorhies Then & Now!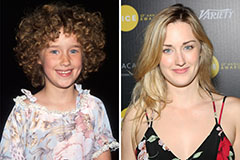 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Bernadette Peters Then & Now!
Bernadette Peters Then & Now!